Q&A: GammaTile® brachytherapy for brain tumors with Dr. Vincent DiNapoli 
What is GammaTile?
GammaTile is a targeted radiation therapy used to prevent recurrence after a brain tumor is removed. GammaTiles are thin sponges holding seeds that deliver radiation to the tumor cavity. The therapy was approved in 2018 by the U.S. Food and Drug Administration for the treatment of recurrent brain tumors, and in 2020 for newly diagnosed malignant brain tumors. GammaTile is covered by Medicare and most other insurance carriers.
How does GammaTile work?
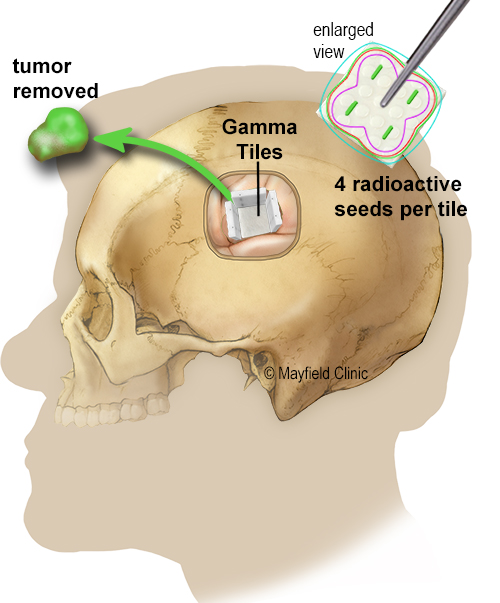
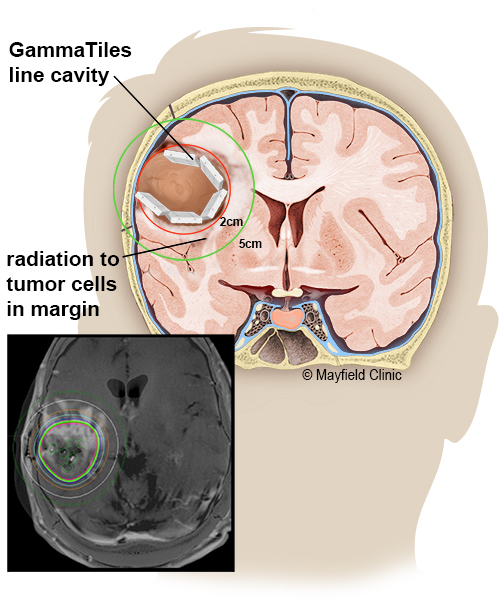
GammaTiles are implanted during the final stages of brain tumor surgery. The surgeon places the collagen tiles inside the tumor cavity immediately after removing the tumor. During the next six weeks, they deliver radiation to any residual tumor cells before they can replicate. It is an innovative form of brachytherapy, where the source of radiation is placed near or inside a tumor. Once the seeds are no longer radioactive, the tiles remain in the brain and are eventually absorbed.
What types of brain tumors is GammaTile used to treat?
Patients with operable malignant brain tumors are eligible for GammaTile, including:
• Newly diagnosed glioblastoma (GBM)
• Recurrent gliomas & GBMs
• Brain metastases (cancer in other parts of the body that has spread to the brain)
• Recurrent malignant meningiomas
What are the advantages of GammaTile?
GammaTile helps preserve healthy brain tissue. Radiation therapy starts quickly, so patients don't have to wait to heal from surgery. This delivery method provides patients with an opportunity to complete the entire spectrum of care for that particular tumor in one episode, rather than having to come back to the hospital for multiple external radiation sessions.
What's the difference between GammaTile and Gamma Knife radiosurgery?
Gamma Knife radiosurgery uses radiation aimed at the brain from outside the body. Nearly 200 low-dose and high-energy beams converge at a single point to kill targeted cells while avoiding damage to nearby healthy cells. Gamma Knife is an outpatient procedure. GammaTile, by contrast, is used in conjunction with traditional surgery to disperse radiation from inside the brain cavity after the surgeon has removed a tumor. The radiation from GammaTile can avoid the need for additional radiation treatment.
What does the latest research show about the safety of GammaTile?
Mayfield Brain & Spine is one of the nation's leading GammaTile centers and offers the newest clinical trials. Most recently, Mayfield served as one of 12 sites across the country, and the only site in Ohio, for a clinical trial related to brain metastases, or cancer that spreads from other areas of the body, such as the lung or breast. According to a report in the journal Neuro-Oncology Advances, the study showed no adverse events directly attributable to GammaTile, and concluded that GammaTile is a safe option for treating newly diagnosed brain metastases.
YouTube: Watch a patient describe her experience with GammaTile®
For more information about GammaTile clinical outcomes, visit GammaTile.com
Are there any GammaTile clinical trials at Mayfield?
Yes, for more information on the study and if you qualify, visit our clinical trials page.
- ROADS Study: GammaTile vs. Stereotactic Radiotherapy for Newly-Diagnosed Metastatic Brain Tumors
- GammaTile, STaRT Observational Study in Neoplasms.


